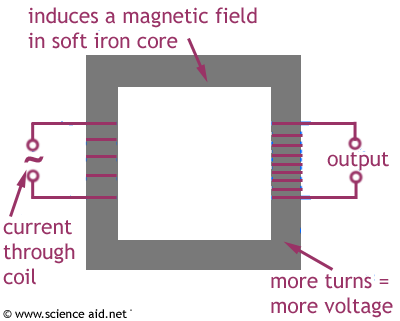
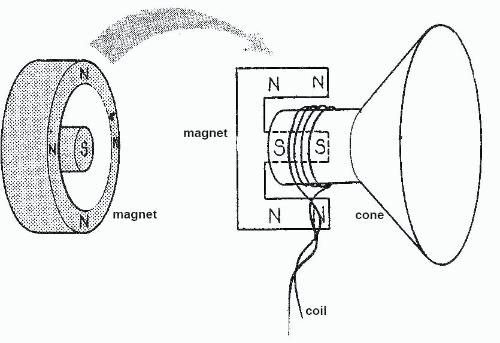
Hopefully people will makes use of this, whilst also remembering that I may have made mistakes (please feel free to correct me.)
Good luck to everyone!
If I make any resources I may share them...
In the mean time here are some of the resources that I have used when making my notes:
- http://www.gcsescience.com/index.html
- http://www.physics.isu.edu/radinf/risk.htm
- http://www.animations.physics.unsw.edu.au/mechanics/
- http://www.magnet.fsu.edu/education/tutorials/java/magwire/index.html
- http://www.youtube.com/playlist?list=PL9C873D6281FA9535
- http://www.youtube.com/user/TutorVista?feature=
- http://www.youtube.com/user/scienceteacher09?feature=
- http://www.youtube.com/user/PelletierPhysics?feature=
- http://www.youtube.com/user/PhysicsEH?feature=
- http://www.youtube.com/user/highschphysics?feature=