Nuclear equations show the atoms atomic number and atomic mass on one side and the radiation (with mass and number) and the new element (with mass and number.)
For example, uranium has experienced alpha decay:
 |
| thinkquest |
Here lithium has experienced beta decay:
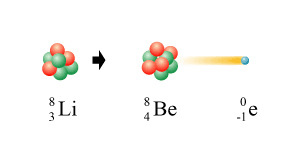 |
| hk-phy.org |

Hey Hannah, Thank you so much for this blog.
ReplyDeleteMoreover, there is a mistake, in beta decay we add one to the atomic number.Further its not +1 in e, its -1 therefore we add one..
I hope you get this.. :)
Hi, thankyou! I looked through radiation and corrected a few mistakes; I'm not sure if I changed the one you highlighted though?
Deleteheye.. yes i am sure, it is the mistake..
ReplyDeleteLet me give the source..
http://en.wikipedia.org/wiki/Beta_decay
Thanks.
Oh I see the mistake! I have changed it I think :) Thank you so much, I would never have spotted that! Thank you :)
Delete:)
ReplyDeleteThanks for ur hard work
ReplyDeleteu saved my career